Medical Device Software Development: What You Need to Know

Medical device software now drives the core of digital health. AI powers diagnostics, IoT connects patients to providers, and real-time data shifts care from reactive to predictive. According to recent statistics, the global SaMD market is projected to grow from $2.77 billion in 2024 to $52.87 billion by 2033 at a compound annual growth rate (CAGR) of 38.4%. The U.S. advances rapidly with FDA pathways designed for speed, while Europe maintains a GDPR-compliant digital health infrastructure, holding a 30% global market share.
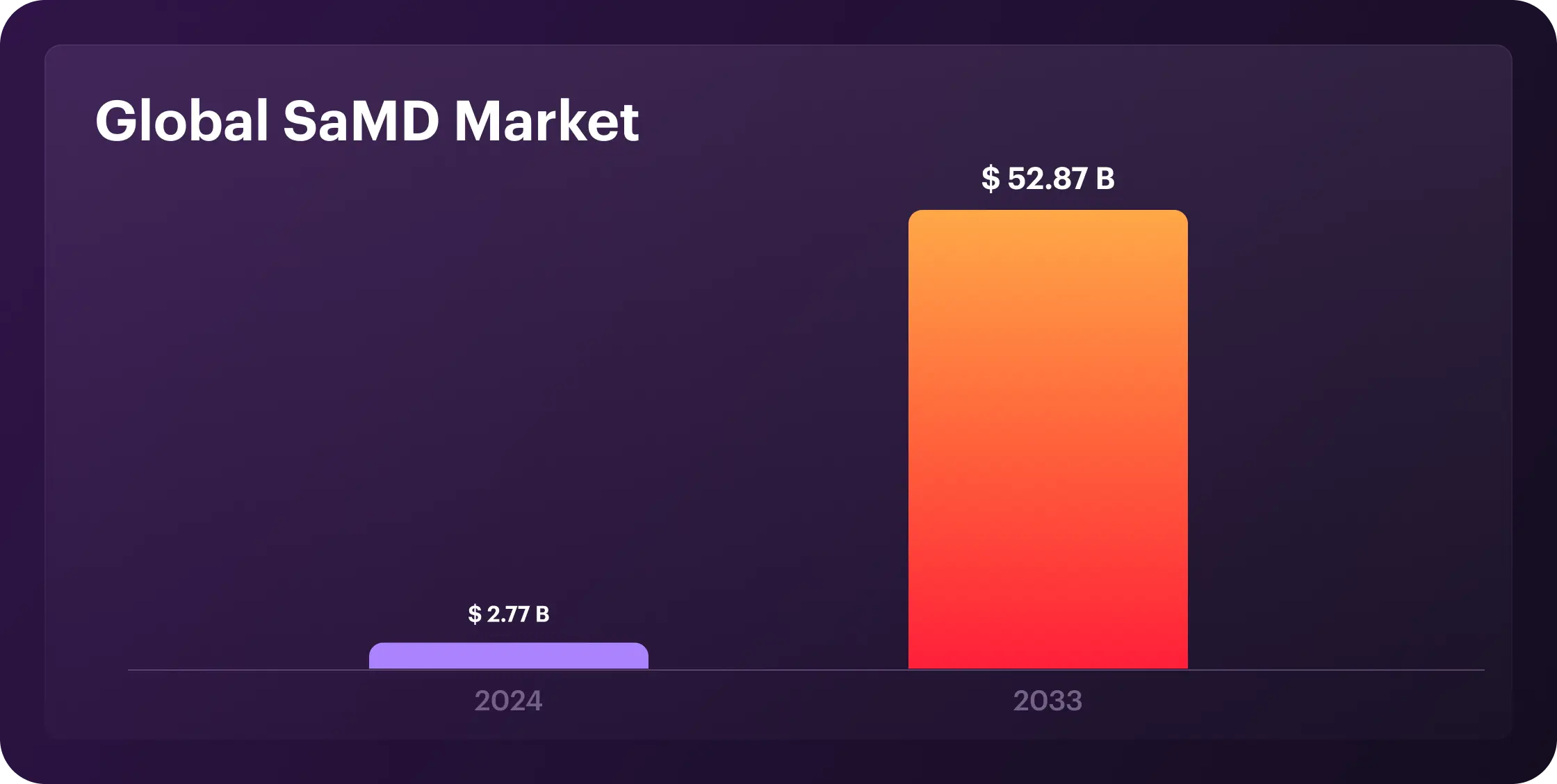
Nevertheless, the future is bright for SaMD; it has benefits and challenges. Let's dive into the meaning of medical device software development, including the steps and types.
What Is Medical Device Software Development?
Medical device software development refers to designing, creating, testing, and maintaining software that is integral to the functionality of medical devices or operates as a standalone medical device. This software must meet strict regulations for safety and accuracy, including those from the FDA, MDR, and ISO 13485.
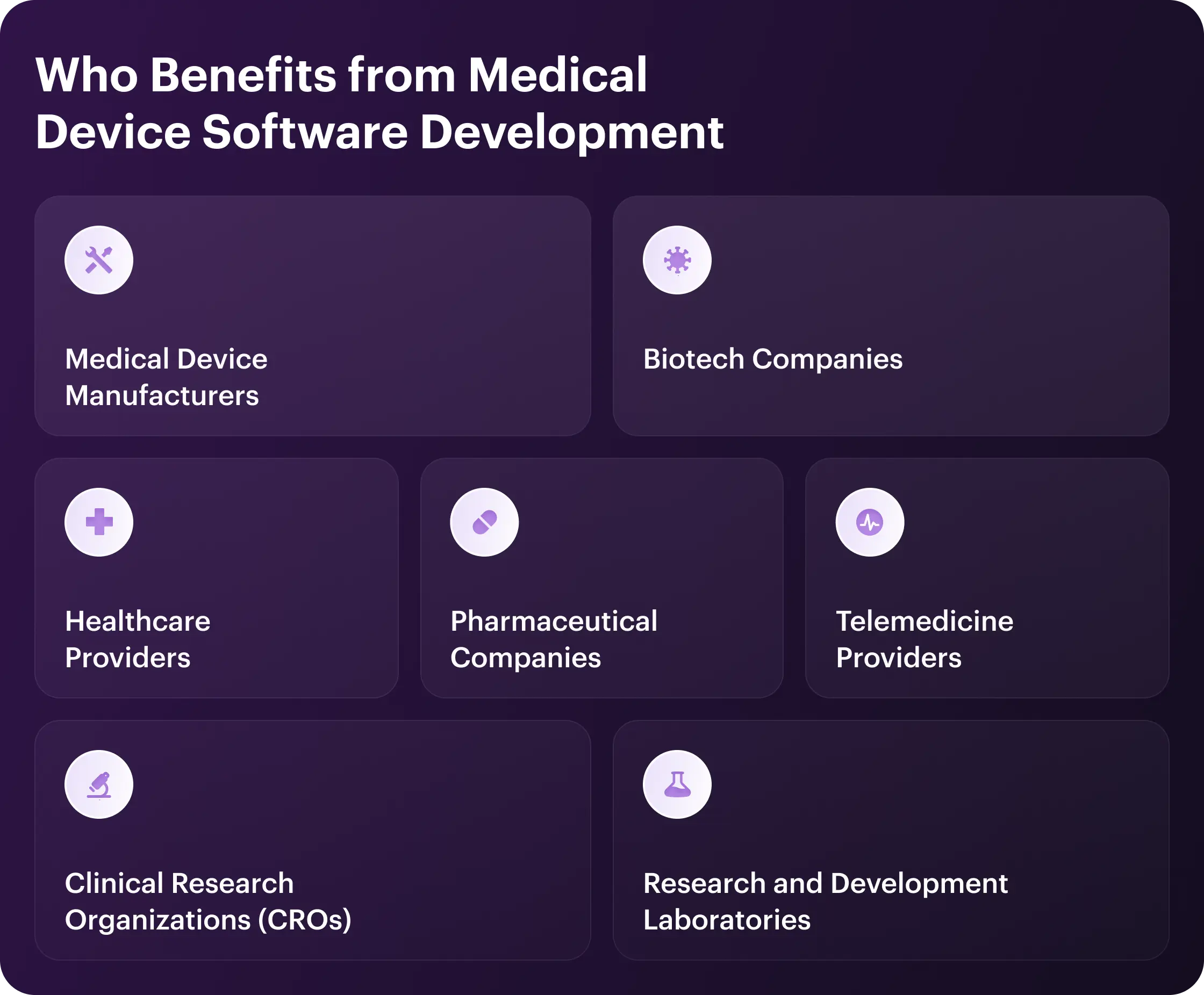
Who Benefits from Medical Device Software Development
Demand for medical device software spans many sectors. Let's explore the industries leading the way.
Healthcare Providers
Real-time patient data helps providers make sharper decisions and diagnoses. Automation reduces manual errors and allows staff to focus on care. AI and IoT add efficiency and enable remote, personalized treatment. This delivers faster, safer care while cutting costs.
Medical Device Manufacturers
Medical device manufacturers use software to automate quality control and ensure compliance with regulations. Real-time monitoring reduces errors and helps make proactive adjustments. Scalable updates future-proof devices, while ERP systems improve supply chains, reduce costs, and accelerate time-to-market.
Pharmaceutical Companies
Pharma firms cut trial costs via wearables, automate drug production with digital batch records, and monetize AI-powered SaMD apps that improve adherence. Real-time monitoring speeds up approvals, while AI-driven data tailors therapies and unlocks revenue from digital treatments and SaaS models, sharpening efficiency and market edge.
Telemedicine Providers
Telemedicine thrives in video conferencing and remote monitoring, particularly in underserved areas. Yet, it needs specialized software to connect with medical devices for remote diagnosis, monitoring, and treatment. Modern medical device software improves access to care, diagnostic accuracy, patient engagement, and chronic disease management while also helping reduce hospital readmissions.
Biotech Companies
Biotech companies use medical device software to process complex biological data, speed up drug discovery, and perform diagnostics. Custom tools automate tasks while ensuring compliance with FDA and HIPAA regulations, reducing risk. The result? This tech drives innovation, cuts costs, and accelerates the development of market-ready biotech products.
Clinical Research Organizations (CROs)
CROs gain efficiency and accuracy through automated data handling in device trials. Better data management and real-time monitoring improve trial quality and speed. This leads to faster market entry, reduced costs, and ensured regulatory compliance.
Research and Development Laboratories
Medical device software transforms R&D labs. Thus, teams automate tasks, use AI for sharper data analysis, and lock in compliance from day one. As a result, labs hit faster diagnostics, therapies, and disease control breakthroughs without slowdowns.
Advantages of Software Development for Medical Devices
Software is the new heartbeat of healthcare, making devices smarter, faster, and more efficient. Better outcomes and lower costs are just the beginning. Discover the full positive impact below.
Increased diagnostic accuracy
Medical device software improves diagnostic precision through advanced data analysis techniques. Algorithms detect subtle patterns humans might miss, especially in imaging and laboratory results. Machine learning models continually refine their accuracy by analyzing millions of patient cases, significantly reducing error rates.
In practice, whole-slide imaging in digital pathology achieves a diagnostic concordance of 96.5%, matching that of traditional light microscopy. AI models like GPT-4 show pooled diagnostic accuracy of 52.1%, on par with non-expert physicians but still below experts by 15.8%
Process automation
Automation eliminates repetitive tasks, reduces errors, and allows medical staff to focus on patients. The software handles everything from scheduling to medication management, reducing costs while boosting efficiency. Hospitals report significant time savings and happier staff after implementing automated systems.
Real-time patient monitoring
Connected devices track vital signs without constant staff presence. The software alerts doctors when readings breach safe thresholds for immediate intervention. This capability saves lives in ICUs and helps patients with unpredictable chronic conditions.
Integration with medical systems
Medical software integrates with hospital databases, electronic health records, and laboratory systems. This eliminates data silos and ensures all providers see complete patient information. System interoperability reduces duplicate data entry and lowers the risk of transcription errors.
For instance, integrated medical software reduces duplicate data entry by 30% and transcription errors by over 50%. Hospitals with connected EHR and lab systems report a 25% boost in care coordination and faster clinical decisions.
Regulatory compliance
Built-in audit trails, access controls, and encryption protect patient data while meeting legal medical device software standards. These safeguards prevent costly violations and data breaches that damage trust and finances.
Flexibility and scalability
Medical software adapts to changing needs through modular design and cloud deployment. Solutions scale from single clinics to hospital networks without performance loss. Providers start with core functions and expand as requirements evolve.
Remote access and telemedicine
Telemedicine breaks geographical barriers to healthcare delivery. Remote monitoring enables home recovery while maintaining professional oversight. These systems maintain care continuity during emergencies when facilities reach capacity.
Cost reduction
Software-driven automation delivers significant savings through improved efficiency. Predictive maintenance prevents equipment failures and reduces downtime. Digital transformation reduces administrative overhead, with hospitals reporting a 30% decrease in operational costs.
Improved user experience
Intuitive interfaces cut training needs and boost adoption among medical staff. Human-centered medical device software design makes complex technology accessible to everyone. Patient apps improve treatment adherence with simple instructions and timely reminders.
Support for analytics and AI
Analytics transform medical data into actionable insights for treatment. AI identifies high-risk patients before acute episodes occur. Machine learning models continually improve with new clinical data, keeping recommendations updated with medical advances.

Key Aspects to Consider in Medical Device Software Development
Medical device software revolutionizes healthcare, but building it is no walk in the park. From regulations and compatibility to testing and ongoing maintenance, the stakes are high, and the margin for error is zero in medical device software development. Here's what matters most and saves lives.
Meeting Regulatory Standards
Regulatory compliance is non-negotiable. Developers must navigate a maze of standards from bodies like the FDA or EMA, which classify software based on risk and dictate the approval process. Whether it's a 510(k) or a PMA, getting them wrong can result in costly delays or outright rejection. Stay updated, plan for compliance from day one, and document everything—regulators love paperwork.
Ensuring Data Security and Privacy
Medical software handles sensitive patient data, which makes security a top priority. Encryption, secure authentication, and regular audits are must-haves to protect against breaches. HIPAA and GDPR aren't just guidelines—they're the law. One misstep can lead to hefty fines and a PR nightmare, so lock it down.
Designing for Usability and User-Centered Experience
If your software design for medical devices isn't user-friendly, it's a liability. Engage with doctors, nurses, or patients early and often to design interfaces that reduce errors and fit seamlessly into their workflows. Accessibility isn't optional—it's essential. A confusing UI can lead to misdiagnoses or delays, so make it intuitive and easy to use.
Ensuring Compatibility with Healthcare Systems
The software never lives in a vacuum. It must comply with EHRs, LIS, or PACS, using standards like HL7 or DICOM. Since interoperability isn't just a buzzword—it's how data flows smoothly in healthcare—build with flexibility in mind. Systems change, and your software should keep up.
Implementing Effective Risk Mitigation
Risk management is baked into medical software development. Use frameworks like ISO 14971 to identify hazards, assess risks, and implement controls to manage them. Plus, tools like FMEA help catch issues before they become disasters. In this field, an ounce of prevention is worth a pound of cure.
Thorough Testing and Quality Validation
From unit tests to clinical validation, every layer counts. Rigorous validation, covering unit, integration, system, and user acceptance testing, helps catch defects early. Include performance, security, and interoperability checks. Pay attention to documenting everything: regulators will demand proof.
Ongoing Maintenance and Software Enhancement
Launch day is just the beginning. Bugs, security patches, and new features require constant attention. Set up a robust post-market surveillance plan to catch issues early. Your software should evolve with healthcare, not fall behind.
Types of Medical Device Software
Based on hardware and regulatory integration, medical device software falls into two primary categories:
Embedded Software for Medical Systems (EMSSW)
This type of software is an integral part of medical devices, such as pacemakers, infusion pumps, and imaging machines. Embedded systems control device functions, ensuring they operate safely and effectively. It is not standalone software like desktop or mobile apps, and it cannot function without the hardware of the medical device.
Examples of Embedded Software for Medical Systems (EMSSW) are:
- Pulse oximeters.
- Smart bio-sensors.
- Glucometers.
- Electronic blood pressure sensors.
- Medical imaging devices, such as X-rays, MRIs, and CT scans.
Standalone Software as a Medical Device (SaMD)
Standalone Software as a Medical Device (SaMD) performs a medical function independently of dedicated hardware. Think algorithms spotting heart issues in ECGs or apps managing diabetes. Regulated by the FDA and EU MDR, it operates on phones, laptops, or clouds, slashing costs and supercharging care delivery.
Examples of Standalone Software as a Medical Device (SaMD):
- Patient imaging or scan analysis.
- Remote ECG-monitoring.
- MRI Viewing applications.
Steps in Medical Device Software Development
When lives depend on technology, there's no room for error. Follow the steps of medical device software development grounded in timeless principles: safety, usability, and trust.
Comprehend Regulatory Standards
Compliance encompasses global frameworks such as IEC 62304 (software lifecycle management) and ISO 13485 (quality management), as well as regional mandates, including FDA design controls in the U.S. and MDR/IVDR in the EU. Data privacy laws, such as HIPAA in the U.S. and GDPR in the EU, govern the handling of patient information. Developers must integrate these standards early, ensuring risk management, usability testing, and documentation meet audit requirements. A deep understanding of these rules prevents costly delays and ensures software reliability in clinical settings.
Conduct Market Research and Identify User Needs
Research market trends and competitors to define your software's unique value. To gather user needs, engage stakeholders, such as clinicians, patients, and administrators, through interviews or surveys. Translate these into clear use cases and functional requirements aligned with clinical workflows.
Select an Experienced Development Partner
Choose a development partner with proven expertise in medical device software and IEC 62304 compliance. Ensure they understand relevant regulations, such as FDA or EU MDR, and can support submissions and audits. Verify their technical skills in areas such as embedded systems or AI, and confirm that they follow rigorous testing protocols. Prioritize clear communication and strong project management to meet your timeline and goals.
Development and System Integration
Adopt an iterative development approach, such as Agile, tailored to IEC 62304 to balance flexibility and traceability. Design a modular software architecture for scalability and integration with hardware or external systems, such as EHRs, using standards like HL7 or FHIR. Follow secure coding practices and document code for compliance. Integrate software with hardware or APIs, then conduct thorough verification and validation through unit, integration, and system testing to ensure safety and performance.
Deployment and Ongoing Monitoring
Prepare regulatory submissions, such as a 510(k) for the FDA or a Technical File for EU MDR, to gain market approval. Deploy the software in phases, starting with pilot testing in clinical settings to validate real-world performance, train users to ensure proper operation, and minimize errors. Implement post-market surveillance per ISO 13485 to monitor performance, collect feedback, and address adverse events. Use analytics to track usage and identify issues, such as cybersecurity threats.
Ongoing Support and Maintenance
Provide prompt bug fixes and updates to enhance functionality or security, ensuring compliance with regulations for significant changes. Offer user support through helpdesks or chatbots to resolve issues. Plan periodic upgrades to keep up with new clinical needs and technologies. Prepare for software end-of-life by managing data migration and complying with data retention rules.

How to Select the Best Medical Device Software Development Company for Your Project?
First, clearly define your project goals and requirements. List specific features like AI diagnostics or cloud integration, compliance needs (e.g., FDA, EU MDR), and budget constraints. Identify the target users, such as clinicians or patients, to guide usability expectations. This ensures you communicate precise needs to potential vendors.
Second, research companies with a proven track record in medical device software. Check portfolios for projects like remote monitoring or EHR-integrated tools. Review client feedback on platforms like Clutch or KLAS Research. Confirm their experience with regulatory approvals, such as ISO 13485 or CE marking.
Third, evaluate their regulatory expertise. Ask how they handle standards like HIPAA, IEC 62304, or FDA guidelines. Request examples of compliance documentation or successful submissions. Strong regulatory knowledge prevents delays and costly revisions.
Fourth, assess their technical capabilities. Verify expertise in your required tech stack, such as IoT, HL7/FHIR for interoperability, or secure cloud platforms like AWS. Ensure they can integrate with healthcare systems and support cross-platform development. Technical alignment reduces risks and boosts performance.
Fifth, prioritize cybersecurity expertise. Confirm they follow secure medical device software engineering practices like OWASP's S-SDLC and use encryption and authentication. Ask about their process for regular updates and vulnerability patches. Robust security protects patient data and builds trust.
Sixth, check their focus on user-centric design. Review examples of intuitive, compliant interfaces for medical devices. Ensure they conduct UX research to minimize user errors. A user-friendly design improves adoption and care outcomes.
Seventh, confirm their collaboration and transparency. Look for clear communication, detailed project plans, and methodologies such as Agile or Scrum. Request clarity on pricing, timelines, and milestones. Transparent collaboration aligns the project with your vision.
Eighth, compare costs while prioritizing value. Expect custom medical software to cost between $ 200,000 and $400,000, depending on its complexity. Ensure they offer scalable solutions and ongoing support. Choose a vendor that strikes a balance between quality and budget without compromising on standards.
Finally, shortlist 3-5 companies and request detailed proposals from them. Conduct interviews to assess their approach, certifications, and alignment with your goals. Select the partner with the best mix of expertise, reliability, and transparency. Sign a contract only after reviewing terms and ensuring mutual clarity.
The Future of Medical Device Software Development
Medical device software already stands at the cutting edge of healthcare innovation, driving massive change in how we diagnose and treat patients. Yet, with billions of dollars flowing into health tech and regulatory barriers easing, the future of medical device software development is bright for the coming years.
AI and Machine Learning Integration
AI algorithms are already making waves in the medical device software sector. Predictive analytics and personalized treatments help spot patterns humans miss, delivering faster diagnoses and better outcomes. The tech now works across nearly every medical specialty, and the trends show it won't stop soon, only evolve.
Cybersecurity as Priority
Security doesn't leave the center stage as connected medical devices face sophisticated threats. Manufacturers now build in encryption, strong authentication, and continuous security monitoring from day one. Patient data protection matters as much as clinical functionality.
Edge Computing Implementation
Processing moves to the device itself, shortening wait times for essential procedures. The tech works even when networks fail, and it is a game-changer for implantables and wearables. Edge computing delivers real-time insights exactly when patients need them.
Interoperability Standards
The industry finally tackles its fragmentation problem with standards that work. FHIR and open APIs create true connectivity between previously siloed systems. The seamless data flow means better decisions based on complete patient records.
Sustainability and Scalability
Developers focus on energy-efficient software to extend the battery life of devices. Modular designs allow easy updates without replacing hardware. Cloud integration supports scaling for large patient populations. This reduces costs and environmental impact.
Regulatory Evolution
Regulators are finally catching up to tech innovation with more flexible frameworks. The FDA and international bodies now offer pre-certification pathways and real-world performance monitoring. Companies can innovate faster while still proving their products are safe.
Why Darly Solutions is Your Best Medical Device Software Partner?
No cookie-cutter code here. We're healthcare tech experts who solve real problems. For hospitals, clinics, and MedTech startups, we've delivered over 60 projects that handle messy data, strict compliance, and seamless integration.
Every solution is tailored, whether you're upgrading old systems or launching new ones. Our tech stack is healthcare-tested: secure, compliant, and built to last. Here's how we do it:
Our services cover:
- Custom software development for unique workflows.
- App development that works across devices.
- Cross-platform solutions to reach more patients.
- Automation services to slash admin time.
- UI/UX design that the medical staff actually uses.
- Integration services to connect legacy systems.
We build dashboards that act, not just display. Our scheduling tools cut admin work in half while keeping patients happy. Need e-prescribing or billing that's both secure and fast? We've done it reliably on a large scale.
Need experts who speak healthcare? Whether it's a whole managed IT team or project-specific help, we're here. Outsource your software development to us, and let's build tools that save time, money, and lives.
Conclusion
Medical device software development is more than code—it's about building tools that make healthcare smarter, safer, and faster. With the right partner, you get solutions that boost outcomes, cut costs, and keep you ahead of the curve. Darly Solutions brings deep healthcare expertise, proven results, and a relentless focus on real-world impact. If you're ready to transform your healthcare business, Darly Solutions is the team to trust.
FAQ
Medical device software costs range from $ 100,000 to $500,000+ for basic systems and over $1 million for complex ones. Key cost drivers include design ($20,000–$40,000), development ($60,000–$80,000), testing ($10,000–$15,000), and annual ongoing maintenance ($3,000–$5,000).
From strict regulatory compliance (FDA/IEC 62304), cybersecurity risks, and integrating new tech with legacy systems to balancing safety with usability, medical device software development might take it all.
Medical device software development uses embedded languages (C/C++, Python) for core functions, cloud platforms (AWS, Azure) for data/connectivity, and security tools (encryption, HIPAA compliance) for safety.
Connect with us
.webp)
We are a tech partner that delivers ingenious digital solutions, engineering and vertical services for industry leaders powered by vetted talents.





.webp)