Why Healthcare App Time to Market Matters: The Risks of Delays in Medical App Launch

Healthcare apps have already become a center of explosive growth and a digital battleground. Patients demand high-quality on-demand care, providers seek seamless data integration, and dev teams need faster time to market software development cycles.
According to industry insights by Statista, there will be over 2.6 bn active healthcare app users worldwide by 2029. In such a fast-moving environment, healthcare app time to market goes far beyond a performance metric. It’s the difference between capturing early adopters and watching them sign up elsewhere.
Getting an MVP for medical apps early makes it possible for dev teams to validate assumptions, refine features and workflows based on real clinician feedback and budget. But many teams get stuck in the transition from a minimum viable product (MVP) to a “fully featured” solution. They are adding every requested widget, focusing on non-essential UX enhancements, or pausing for prolonged compliance sign-offs. This can lead to growing technical debt and missed opportunities – while more agile newcomers seize the opportunity.
Add some peculiarities of the healthcare (HIPAA and GDPR reviews, integrations with multiple EHR vendors, and approvals of clinical stakeholders) – and teams have a recipe for release delay. Every extra day spent polishing secondary functionality is a day competitors use to build brand loyalty and collect invaluable usage data. This article uncovers the top 5 risks of delayed app development in healthcare and shows how you can keep ahead of challenges and competitors.
What Is Time-to-Market (TTM) in Healthcare App Development?
Healthcare app time to market refers to the total duration from ideation (validating a medical or operational need) to deploying a fully compliant, tested app into the hands of users (clinicians or patients). A well-thought healthcare app go-to-market strategy with app validation and approval ensures you hit that window without sacrificing quality.
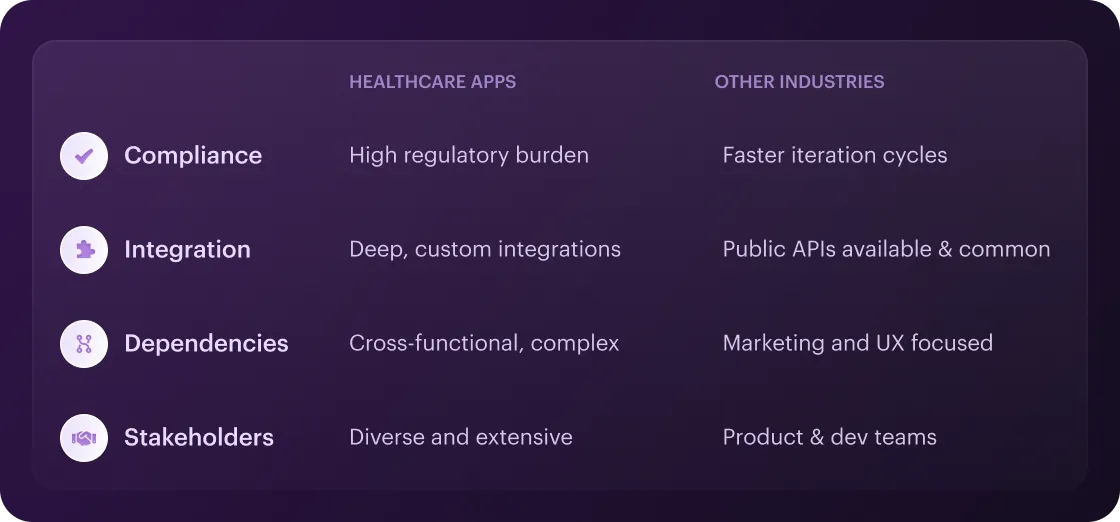
Key Differences vs. Other Industries
- Compliance vs. Speed. Most of the apps often iterate weekly, but healthcare apps must build in audit trails, data privacy checks, HIPAA compliance, and clinical validation before any release.
- Integration Depth. Unlike other apps that may rely on public APIs, healthcare solutions need custom-made connections to EHRs, lab systems, and medical devices. This extends development cycles.
- Cross-Functional Dependencies. In consumer tech, feedback loops involve marketing and UX. In HealthTech, there are clinical trials, IRB sign-off, and legal counsel checkpoints – each adding gating milestones.
- Stakeholder Complexity. In other industries, product managers and dev teams dominate. In healthcare, you also need C-suite buy-in, clinician endorsement, and often patient advisory boards.
The Importance of Time to Market in Healthcare App Development
Early adopters gain loyalty among clinicians and patients who trust and advocate for your solution. Shorter TTM means you gather real usage data sooner – critical for agile healthcare development and updating your product roadmap based on real feedback. With hundreds of digital health startups ready to release – you should have a clear time to market healthcare app roadmap. So that you leave less room for rushing competitors.
5 Critical Risks of Delayed App Development in Healthcare
Delaying your healthcare app launch isn’t just inconvenient and budget-consuming. It carries real consequences. Below, we’ll explore the top 5 delay risks in medical app launch. From losing your competitive edge and shorter lifetime value – to greater compliance exposure, growing costs, and users who simply move on.
Loss of Competitive Advantage in the Healthcare Market
Delays in medical app launch aren’t merely setbacks. They’re strategic obstacles that silence your brand, impact your revenue, and even completely shift partnerships to more reliable development teams. Here’s what’s at risk when your launch date slips.
- Stalled Brand Recognition. A late entry means fewer press mentions, conference slots, and speaking invites. While competitors share success stories, your brand remains silent – and silent brands get forgotten.
- Eroded ROI. Every week you miss reduces your window to capture early adopters and referral volume. Lower adoption at launch also means diminished lifetime value and ROI.
- Lost Partnerships. Hospitals, payors, and OEMs lock in partnerships based on roadmap commitments. When you stall, they reallocate budgets to faster, more reliable teams – making it much harder to win those deals later.
For example, during the initial COVID-19 surge, one telehealth startup planned a Q2 launch – but slipped into Q4. By then, larger platforms had secured major health system contracts and patient subscriptions. And they will leave the latecomer fighting for niche use cases and just modest market share.
“Teams should focus on the killer feature. Lunch with what solves the biggest pain point first, and then you can easily layer on secondary modules. Also, engage compliance experts early to clear regulatory hurdles in parallel paths (for non-critical features) – and avoid gating the entire release ” – Bogdan Paiuk, Head of Delivery
Reduced ROI and LTV for Medical Applications
Delays in medical app launch and quality assurance delays don’t just push your release date. Low speed to market in digital health shortens the entire revenue curve. Every week spent polishing secondary features is a week cut off from your app’s lifecycle, reducing both return on investment and Customer Lifetime Value (LTV).
- Fewer Billing Cycles. Late launches mean fewer subscription renewals or usage-based invoices over the product’s lifetime. This directly impacts ROI.
- Slower Feedback-Driven Upsells. You lose precious time to validate upsell opportunities (advanced analytics, premium support), resulting in impacted LTV growth.
- Accelerated Depreciation. Medical apps must evolve to stay compliant and competitive. Delayed starts compress your window to recoup development costs – before major platform changes or new regulations force refactoring.
- Increased Churn Risk. Users who perceive your app as “late to market” often question its viability. This lowers engagement and further shortens LTV.
For example, a tele-rehab solution missed its pilot launch by three quarters. The surprise delay led their hospital partners to delay purchase orders. This will decrease the expected three-year contract value by 25% and trigger further renegotiations at even lower rates.
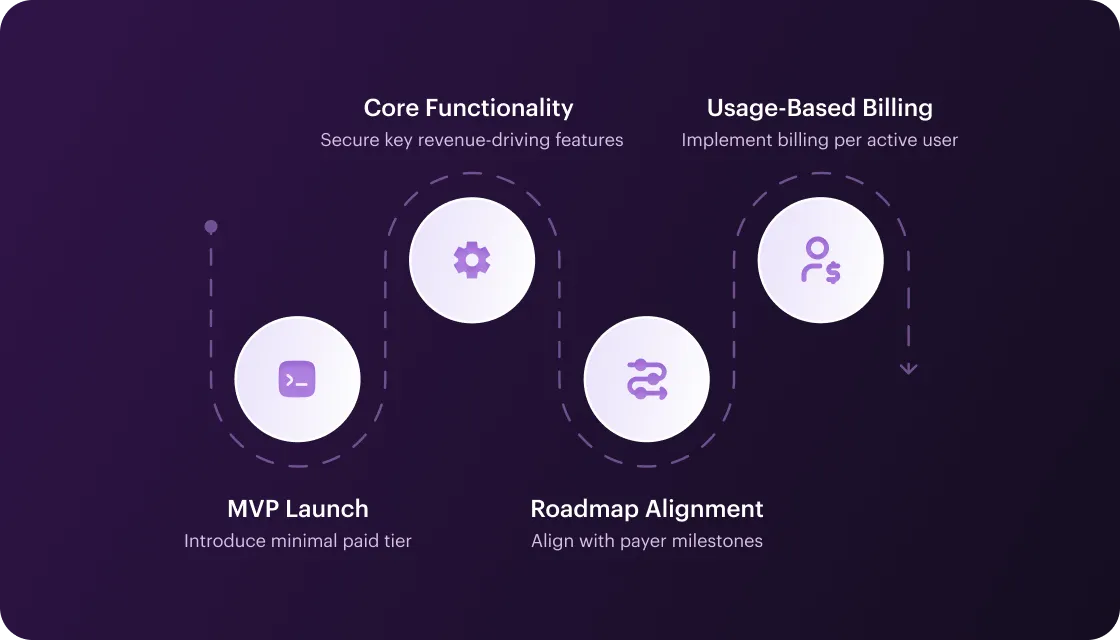
Monetize early – within your healthcare MVP launch strategy, introduce a minimal paid tier (with basic analytics or priority support) to start recouping costs immediately. Lock down core revenue-driving functionality for launch; schedule non-monetized features in later iterations. Align roadmap with payer milestones and implement usage-based billing (you can charge per active patient or session, so even a delayed start yields incremental revenue as soon as users engage).
Increased Risk of Regulatory Delays in Healthcare Apps
When your launch slips, you additionally expose your project to ever-shifting compliance requirements. The longer the development, the more regulations evolve. This can leave you racing to cope with data privacy controls, clinical validation, and audit artifacts.
- Regulation Issues. Bodies like the FDA and EMA periodically introduce new requirements for real-world data collection, long-term safety monitoring, and AI/ML validation. To prevent last-minute surprises, you need a well-planned app launch readiness checklist.
- Fragmented Documentation. Prolonged development without up-to-date trace logs means you’ll struggle to prove design controls, clinical evaluation summaries, or data-handling processes – when regulators come knocking.
- Moving Goalposts. Standards like HIPAA, GDPR, FDA’s Software as a Medical Device (SaMD) guidance, and the EU’s MDR frequently update. A six-month delay can mean completely reworking user consent flows or risk assessments.
- Audit Burnout. Legacy code written under yesterday’s rules often lacks the documentation and test coverage needed for today’s inspections. This often leads to last-minute surprises in your go-live strategy or even failed audits.
- Budget Overruns for Compliance. Unplanned compliance work (penetration tests, updated security protocols, new clinical studies) can easily elevate post-launch costs by 20–30% and even more.
For example, a remote-monitoring app under development paused for an extended MVP enhancement cycle. During that window, their intended market in the EU adopted new MDR rules. The team will have to redesign device-classification logic, conduct fresh risk analyses, and re-submit to notified bodies. This will add several months and formidable unplanned expenses.
We recommend embedding hard cut-offs in your roadmap (after a certain date, only critical compliance updates go in – so you at least focus on a stable target for audits). Reserve part of each sprint (for example, 15%) exclusively for regulatory deliverables. And, to prevent regulatory delays in healthcare apps from day 1, architect your system so that data privacy, encryption, and consent modules can be updated independently, minimizing retrofits.
Rising Development and Opportunity Costs for Health Apps
When your healthcare app stalls, every extra sprint directly drains the budget and further exposes resource bottlenecks. Extended healthcare app time to market translates directly into higher vendor fees, engineering salaries, and cloud expenses – without delivering new value. And remember about the revenue you could’ve earned, partnerships you missed, and user insights that may have been left untapped.
- Prolonged Development. As scope creeps and unplanned compliance work stacks up, teams stay endlessly in build mode – with little results to show for it.
- Budget Overruns. Unanticipated QA cycles, additional security audits, and rework can easily raise costs by up to 20–40%. This will be squeezing funds from other areas, for example, marketing or further R&D.
- Resource Drain. Key developers remain tied up in legacy code fixes. This will prevent you from exploring adjacent innovations like AI-driven analytics or patient engagement features.
- Innovation Stagnation. Dev teams focus on existing scopes, giving up exploratory work on breakthrough features that could differentiate your product with greater efficiency.
For example, a digital therapeutics startup planned a six-month healthcare MVP launch strategy – but ended up in a 12-month cycle due to feature bloat and regulatory retesting. Their initial budget will then be doubled. And by launch, competitors had already captured the lion’s share of their target clinics.
Lock down core functionality for MVP for medical apps and funnel new ideas into a Phase 2 backlog. Schedule compliance deliverables in parallel “compliance sprints” to avoid impacting feature work. Reserve 15% of your budget and health app development timeline for unplanned tasks (so overruns don’t threaten your entire project). And you can also go for financial impact modeling – simply tie each week of delay to revenue forecasts and marketing ROI (so decision-makers will understand the true cost of postponement).

User Fatigue and Trust Loss in Medical Solutions
Announcing your healthcare app too early – and then dragging out the release – can exhaust investors, clinicians, and patients. When expectations aren’t met on schedule, initial excitement turns into skepticism.
- Hype vs. Delivery Gap. Don’t set a too high bar – keep away from too early marketing teasers and pilot invitations. The missed health app development timeline disappoints your most engaged prospects and weakens word-of-mouth referrals.
- Churn of Early Adopters. Power users sign up first – and need results fast. When your MVP doesn’t arrive, they abandon the beta and rarely return.
- Reduced Engagement. Users who log in hoping for promised functionality instead encounter a skeleton of features. This inevitably leads to session dropouts, uninstalls, and low retention metrics.
For example, a telehealth startup announced a “smart triage” feature at a national conference. Then it missed its Q2 release window by three quarters. Early users, frustrated by non-functional prototypes, switched back to legacy systems. By the time the feature finally launched, the startup would lose half of its pilot sites – leading to renegotiated contracts at steep discounts.
We recommend releasing to a small, engaged group first. Gather real-world feedback and deliver incremental updates – keeping expectations grounded. If delays occur, proactively update your user base. And only market what’s 80-90% built. Use prototype videos or demo environments to align excitement with reality.
When Delays in Medical App Launch Are Justified
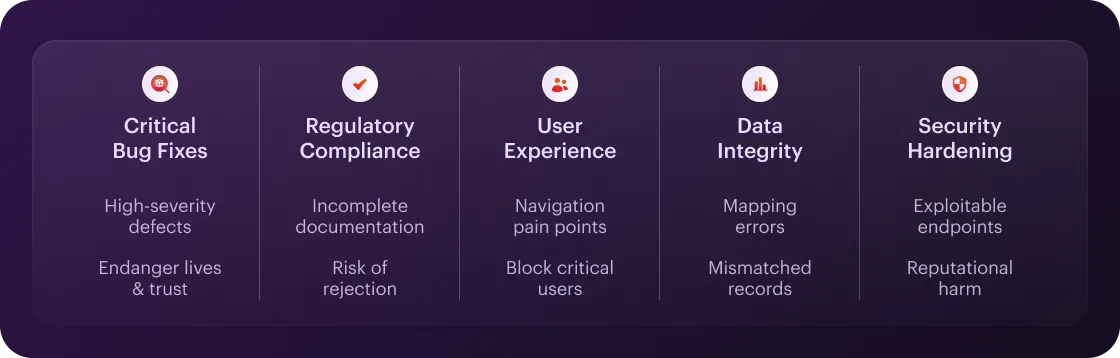
Rapid launches can truly provide great competitive advantages. However, there are times when pausing to perfect is the smarter play – especially in regulated, patient-facing software.
Critical Bug Fixes & Stability
A crash or data corruption in a healthcare app can endanger lives and erode trust instantly. If your QA uncovers high-severity defects (e.g., data loss, security holes, or UI blockers for core workflows), you should widen your testing window before release.
Regulatory Milestones & Compliance Gates
Too early submission to the FDA (SaMD 510(k)), CE marking, or local health authorities can risk rejection (and potentially months of rework). If documentation is incomplete (risk assessments, traceability matrices, validation protocols) or you haven’t passed internal audit checks – postpone the launch until all compliance artifacts are production-ready.
User Experience & Accessibility Readiness
Poorly designed flows (for example, tiny tap targets, unreadable text, missing screen-reader support) will always block every critical user. If usability tests reveal navigation pain points (not to say failures), you should take the time to refine your UI and re-test before roll-out.
Integration & Data Integrity Checks
Healthcare apps almost never run in isolation. EHRs, lab systems, and device APIs must sync flawlessly – or you risk mismatched records or other malfunctions. If end-to-end integration tests catch mapping errors, data latency, or serialization mismatches, fix them before exposing live patients to synchronization issues.
Taking Care of Security
A breach of Protected Health Information (PHI) can result in six- or seven-figure fines and permanent reputational harm. If your pen-testing or vulnerability scans – reveal exploitable endpoints, weak encryption, or improper access controls. You should necessarily extend your remediation sprint until your security posture is 100% bulletproof.
Minimizing Time-to-Market: Proven Practices for Healthcare Apps
Accelerating your launch doesn’t mean cutting corners. It means applying smart, discipline-driven methods that embed both quality and compliance into your go-to-market plan. Here are tried-and-true practices we have been implementing in recent years. Use them to optimize and cut your development cycle.
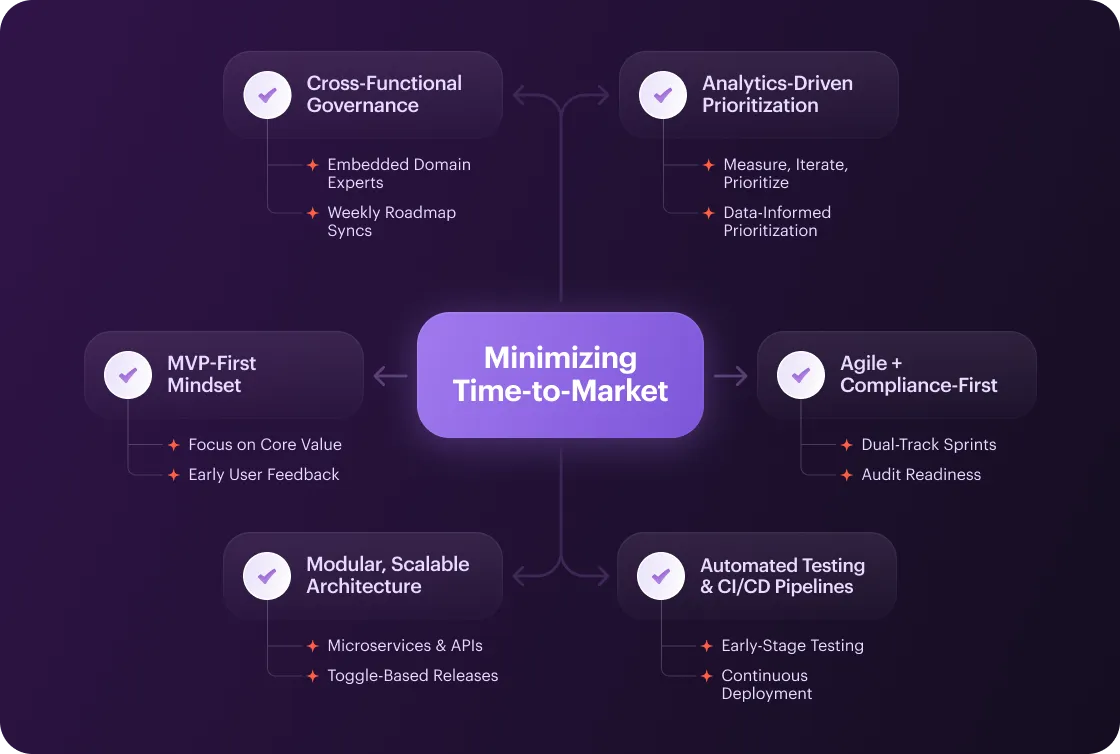
MVP for Medical Apps
- Focus on Core Value. Identify the single most impactful feature (e.g., secure patient login or core measurement dashboard) and release it as your MVP.
- Early User Feedback. Launch to a small pilot group to validate assumptions. You should refine requirements before building non-essential modules.
Modular, Scalable Architecture
- Microservices & APIs. Design components (authentication, data ingestion, notification, etc.) as independent services. That way, you’ll be able to develop, test, and deploy in parallel.
- Toggle-Based Releases. Hide evolving components behind switches, so you can ship the core app and flip features on only when they’ve passed validation.
Agile + Compliance-First
- Dual-Track Sprints. Run feature development and compliance tasks in parallel. Embed security reviews, risk assessments, and documentation into every sprint.
- Definition of Done Includes Audit Readiness. Require that every user story meets regulatory and QA criteria before closing.
Automated Testing & CI/CD Pipelines
- Early-Stage Testing. Embed unit tests, integration tests, and security scans in the CI/CD pipeline from Day 1. Ensure issues are detected and fixed before they snowball.
- Continuous Deployment. Use containerization (Docker/Kubernetes) and automated releases to deploy patches and minor features multiple times per week.
Cross-Functional Squads & Governance
- Embedded Domain Experts. Add clinicians, compliance officers, and UX researchers directly into each agile team for real-time feedback.
- Weekly Roadmap Syncs. Hold a 30-minute governance meeting to review progress, adjust priorities, and unblock decisions.
Analytics-Driven Prioritization
- Measure, Iterate, Prioritize. Track early usage metrics: onboarding completion, feature adoption, etc. Re-prioritize the backlog accordingly.
- Data-Informed Prioritization. Apply usage metrics to determine if delivering a new feature or refining performance should take priority in your next sprint.
Speed to Market in Digital Health: A Competitive Edge
Delays don’t just push launch dates. Low speed to market in digital health can erode LTV, greatly increase development costs, and amplify audit exposure. In healthcare, faster deployment means earlier impact – investments, quicker access to care tools, timely interventions, and reduced administrative friction.
However, a thoughtful delay can be still justified – at least when you employ lean MVP for medical apps, modular design, and integrated compliance. By working on the optimal TTM, you can drive better outcomes and stronger member trust from Day 1.
Need to accelerate your healthcare app time to market – without sacrificing compliance or quality? Want to refine your healthcare app go-to-market strategy? Or feeling ready to deliver functionality without unnecessary wait – and just need some extra development resources, senior or compliance expertise? Book a call and tell us about your project.
FAQ
Connect with us
.webp)
We are a tech partner that delivers ingenious digital solutions, engineering and vertical services for industry leaders powered by vetted talents.







