7 key success factors for digital therapeutics startups


Funding is becoming harder to secure for digital health startups. Q2 2025 marked a five-year low in deal volume across the board, according to a CB Insights report. Digital therapeutics startups aren’t spared: regulatory and approval hurdles, high clinical validation costs, and long sales cycles all make commercialization more complicated than for non-DTx digital health apps.
The EY considers the U.S. regulatory framework for digital therapeutics mature, with Germany, France, Belgium, and the UK following suit. The same report cites a 10% CAGR in global DTx revenue for 2025-2029. Darly Solutions helps digital therapeutics startups build compliant, secure and user-centered products.
Here’s our advice on how to take advantage of the favorable regulatory framework and navigate the industry’s unique funding and commercialization challenges.
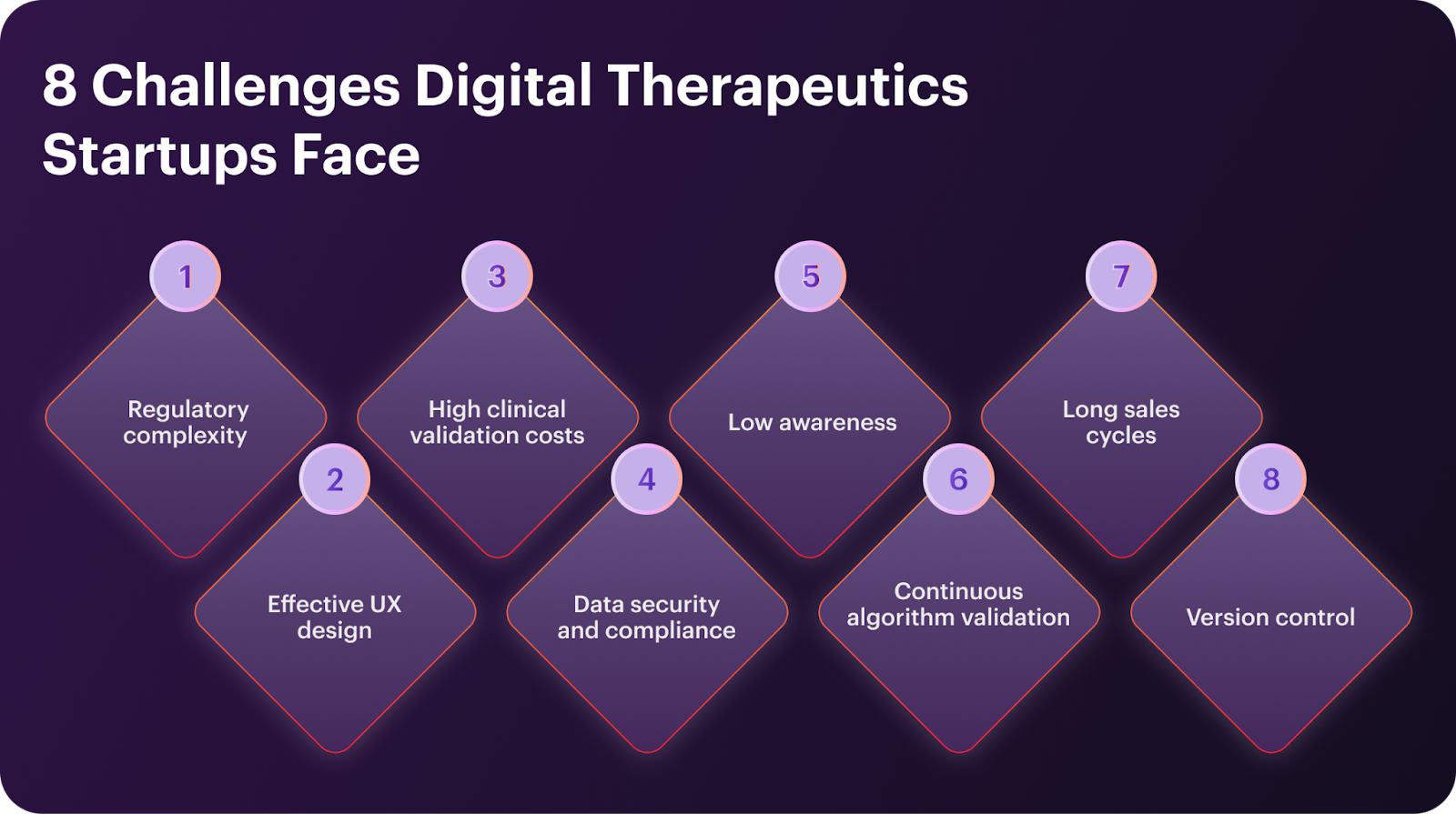
The Challenges Digital Therapeutics Startups Face
Digital therapeutics startups have to grapple with a unique blend of challenges. They share some of them with traditional pharma and medical equipment companies (e.g., clinical validation hurdles). Other challenges stem from their software-only nature, such as scalability and security.
These are the eight key digital therapeutics challenges that may threaten your success:
- Regulatory complexity. DTx products require approval from regulators (e.g., the FDA) and adherence to laws such as HIPAA (U.S.) and DiGA (Germany). Handling the red tape requires substantial overhead, increases risk, and slows down time-to-market.
- High clinical validation costs. Digital therapeutics are evidence-based treatments, meaning clinical validation for DTx (including randomized controlled tests, or RCTs) is a must. Conducting the clinical trials is a significant — and risky — investment.
- Low awareness. Patient and care provider awareness of digital therapeutics remains low. While medication adherence is higher for DTx products than for pharmacotherapy, persuading customers of their clinical value requires investments into market shaping.
- Long sales cycles. For DTx startups that do not market and sell directly to patients, sales cycles last months, as payers and hospitals have complex decision-making processes. Longer sales cycles slow down revenue generation and may deter investors.
- Effective UX design. Much like any medical software, DTx products have to package value-adding functionality into a user-friendly design. The perceived ease of use directly impacts how useful patients find the DTx product. That, in turn, influences their intention to use it.
- Data security and compliance. Health data is extremely sensitive and is regulated as personally identifiable information (PII). Protecting it in line with privacy regulations (HIPAA, GDPR, etc.) requires encryption, robust access controls, and regular security audits.
- Continuous algorithm validation. DTx products that rely on AI/ML algorithms need ongoing model monitoring and maintenance to ensure accuracy and predictability. Algorithm validation also helps maintain high performance and prevent bias and data drift.
- Version control. In healthcare, maintaining an audit trail isn’t optional. So, digital therapeutics products have to log every change to patient data to ensure traceability. At the same time, product changes may require resubmitting it for another approval, stretching out the time-to-market for new features.
The Core Success Factors for DTx Startups
DTx startups that overlook the challenges listed above harm their chances of success. Based on our experience, these seven digital therapeutics success factors help startups successfully overcome them — and secure a path to sustained growth.

1. Clinical Evidence and Real-World Validation
Validating health impact is critical for all patient-facing digital health solutions, but it’s central to any DTx startup strategy. By definition, digital therapeutics are evidence-based interventions. Ergo, you need proof of the product’s clinical value to effectively market and sell it, especially if you target payers and healthcare providers.
Throughout the life cycle, you can aggregate multiple types of evidence, including:
- Clinical evidence. It’s the most common type of evidence obtained before market launch. It typically involves conducting randomized controlled tests, although controlled studies can be used.
- Real-world evidence (RWE). Built upon real-world data (RWD), RWE proves the effectiveness of a DTx product in real-world conditions. Collect the RWD during clinical trials with real-world elements or post-launch.
- Context-specific implementation pilots. These are sources of non-experimental, observational data. Use it to prepare case studies that highlight implementation value.
You’ll need this evidence to demonstrate the product’s clinical value. For clinical trial results to be trustworthy, publish them in peer-reviewed journals. Ensure the evidence covers the following five domains:
- Product safety in the short and long run
- Benefit and effectiveness, as measured by quality of life or other metrics
- Durable treatment effect
- Usability, accessibility, compliance, and adherence to support claims
- Level of user engagement
2. Regulatory and Compliance Strategy from Day One
Unanticipated red tape can easily stretch out the time-to-market. Overlooked compliance requirements, in turn, can threaten your product’s approval or lead to PR nightmares and regulatory fines.
That’s why having a regulatory and compliance strategy is a must for smooth approval, rollout, and operations. To prepare a sound regulatory strategy for DTx:
- Define regulatory requirements before development. Pinpoint all applicable regulations concerning security, privacy, and approval. Translate them into tech specs to ensure alignment from day one.
- Keep global compliance in mind. If you’re planning to expand into multiple markets long-term, unify requirements across jurisdictions. Otherwise, you may need to substantially rework your product to secure approval or introduce localization.
- Choose the right regulatory pathway. Compare different approval pathways (e.g., FDA 510(k), De Novo, and PMA) and understand the tradeoffs in the time-to-market.
- Understand the required clinical evidence. Define the criteria against which your product will be judged. Factor in the time and costs required to conduct clinical trials.
- Settle on the reimbursement pathways. If you choose reimbursements as a revenue stream, study and adapt to the reimbursement pathways. You may need to advocate for your product among payers.
- Define marketing restrictions. DTx products may be subject to advertising rules. Align your business model and marketing strategy with them to avoid non-compliance.
3. Strong Clinical and Industry Partnerships
Partnerships come in many forms, from simple distribution agreements to co-development and revenue-sharing strategic partnerships. Depending on the nature of the partnership, DTx startups can benefit from it in several ways, such as:
- Facilitated access to markets and customers
- Increased product credibility
- Expertise sharing for clinical research, commercialization, etc.
- Additional resources and funding, especially in co-development partnerships
- New distribution channels
When browsing potential partners, consider:
- Pharmaceutical and medical equipment companies
- Insurers and other payers
- Research facilities (universities, hospitals, etc.)
Pharma partnerships for digital therapeutics are especially worth looking into. Pharmaceutical companies are ready to invest in DTx products to address gaps in patient care, improve treatment outcomes, and augment their traditional drug therapies. Cases in point: Sanofi-DarioHealth and Eli Lilly-Sidekick partnerships.
Navigating these partnerships, however, comes with extra challenges. To navigate them successfully:
- Demonstrate the mutual value by highlighting how your strengths complement each other
- Understand and embrace differences in operational workflows
- Adapt the strategy to align with the partner’s objectives
- Decide on the tradeoffs you’re willing to make for a partnership (e.g., sharing revenue)
- Cultivate trust and a close collaboration mindset from the very start
4. User Engagement and Behavioral Design
Successful digital therapeutics products aren’t just built to be clinically effective; they’re designed to promote user engagement to ensure patient adherence. Higher adherence helps demonstrate clinical value to payers, partners, and regulators.
Promoting long-term user engagement in health apps requires paying attention to UX design from the very start of development. Successful UX design is defined by:
- Simplicity and usability. Users with different levels of digital literacy and technical skills should find the product easy to use. Ensure any task can be completed in as few steps as possible.
- Personalization. Leverage patient data to provide tailored health and treatment recommendations and increase the product’s perceived usefulness.
- Accessibility. Add the accessibility standards (e.g., Revised 508 Standards) to tech specs before kicking off development. Those can include voice assistance support, screen reader compatibility, and dyslexia-friendly typography.
- Behavioral design principles. Motivate users to stay engaged with gamification, timely notifications, and social sharing options. Leverage behavioral science principles to guide users with subtle nudges and reinforce behavior with immediate feedback loops.
- Human-centered approach. This approach focuses on aligning the design with the user’s core needs, abilities, and context. Consider complying with guidelines like the ISO 9241-210.
5. Scalable and Secure Tech Infrastructure
Ensuring scalability early on means you won’t need to rebuild the product too soon to accommodate increased performance requirements. It can also help avoid connectivity problems, which are bound to frustrate users.
Core scalability principles to follow are:
- Microservices/modular architecture. Build your product as a collection of interdependent components that can be modified and scaled independently.
- Stateless design. Use this approach to facilitate horizontal scaling later on.
- Cloud-native infrastructure. Leverage serverless tools for performance optimization through load balancing, containerization, and auto-scaling.
Security, on the other hand, is critical for digital health startup success for one simple reason: any digital health product handles sensitive patient data. This data has to be protected against leaks, breaches, and misuse to ensure user privacy.
These six digital therapeutics product development best practices will help you make your product secure:
- Align tech specs with applicable privacy regulations (e.g., HIPAA, GDPR)
- Prepare easy-to-understand privacy notices that explain how you collect, use, and store data
- Secure data at rest and in transit with strong authentication, encryption, and access controls
- Obtain security certifications like SOC 2 and HITRUST
- Continuously monitor internal and external systems to detect incidents early on
- Perform regular security audits, risk assessments, and testing
6. Clear Monetization and Market Access Strategy
DTx commercialization remains a challenge because startups lack an established playbook to guide them. While early examples of successful DTx startups bet on the reimbursement business model, bankruptcies of the likes of Pear Therapeutics put its viability into question.
Needless to say, the monetization strategy is crucial not just for the bottom line but for partners and investors, too. To prepare a successful one:
- Determine how you will differentiate from non-DTx digital health products
- Understand the patient journey and how your product will factor into it
- Define your primary end customer (e.g., patient, payer, healthcare provider)
- Select the most appropriate market access pathways (employee health programs, product reimbursement, etc.)
- Adopt a hybrid business model with primary and secondary revenue streams
As for the market access strategy, outline the key steps for each pathway you select. Here are the available pathways:
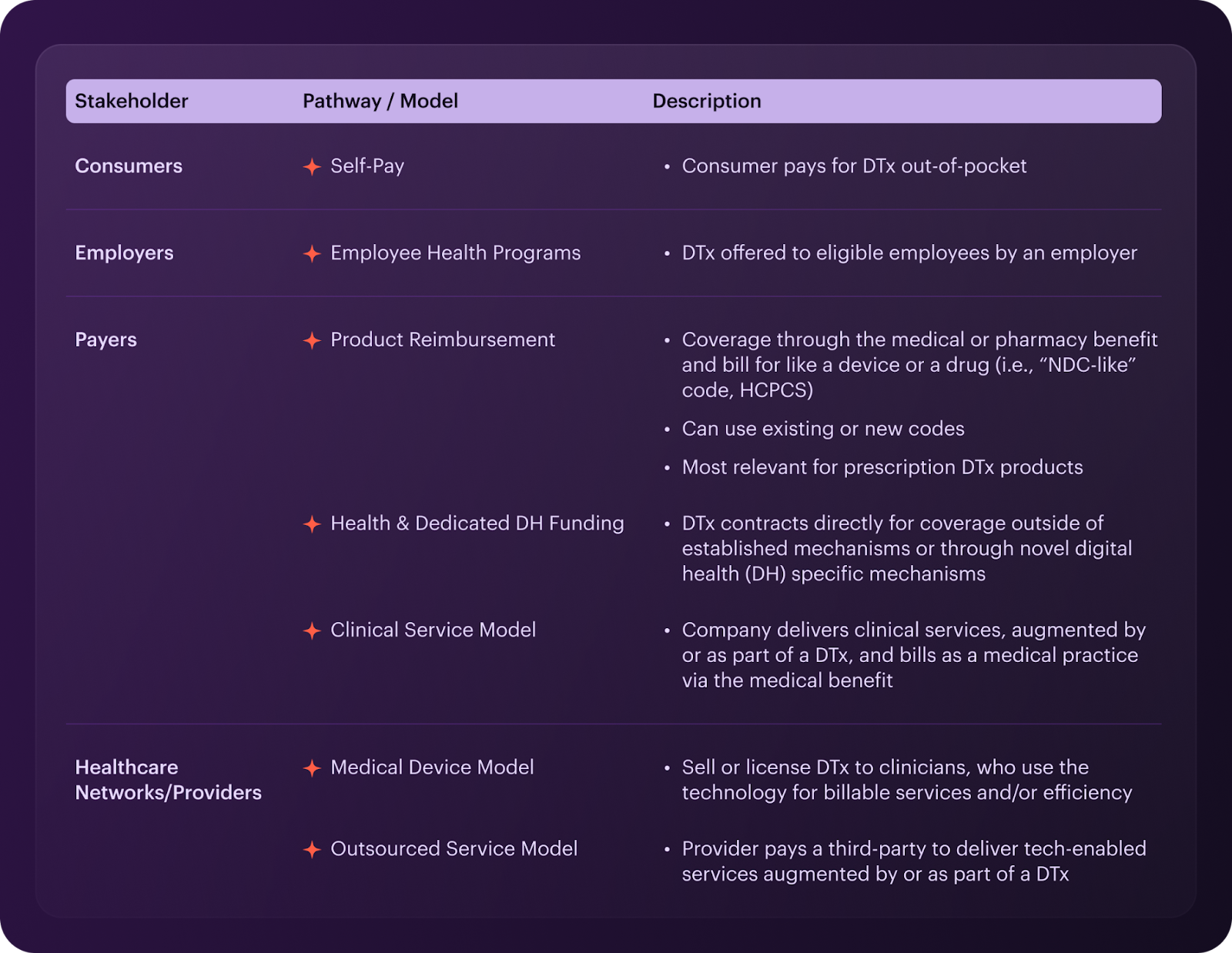
Ensure your market access strategy includes:
- Clear provider and patient segmentation
- Specific routes to market (D2C, pharma partnerships, employers, etc.)
- Comprehensive evidence package for each segment
- Market shaping through stakeholder education
- Easy integration with EMRs to facilitate prescriptions
7. Credible Team and Multidisciplinary Expertise
Health tech innovation always comes with higher risks and lower trust than established products. Therefore, product credibility often hinges on the team's expertise. It can be the decisive factor for partners, investors, and customers alike.
To demonstrate your team’s credibility, put its background, experience, and knowledge front and center. For example, highlight academic credentials or work experience at major companies.
As digital therapeutics products are the amalgam of software and medication, your team should be able to demonstrate expertise on both fronts. That includes experts in:
- The condition your product is treating (IBS, ADHD, etc.)
- Market-specific regulatory and compliance pathways
- Behavioral design and user engagement
- Software security and scalability
- Clinical research
- Data science and AI/ML (if applicable)
Involve other stakeholders during product development to ensure product-market fit, too. Those can include patients, doctors, nurses, social workers, and psychologists.
Emerging Trends That Shape DTx Startup Success
Success factors don’t come only from within the startup. External circumstances can also play to your advantage, provided you seize the opportunity in time. So, pay attention to DTx market trends in 2025 and beyond, such as:
- AI-driven digital biomarkers. Digital biomarkers are signatures that indicate a normal or abnormal disease-related process. AI/ML algorithms power the analysis of the collected data. Digital biomarkers help personalize treatment and patient care, so including them in a DTx product can help drive engagement.
- Integration with connected care ecosystems. Connected care ecosystems strive to weave disparate industry players into a single network. DTx products can join the ecosystem as either treatment or patient data providers.
Key Takeaways
For digital therapeutics startups, success hinges on:
- Clinical value proven by studies and real-world evidence
- Solid regulatory, monetization, and market access strategies
- Human-centered UX design based on the tenets of behavioral science
- Reliable, scalable, and secure software
Need a partner that bakes these success factors into the development process from day one? Darly Solutions will help you unlock growth with engagement-driving UX design, scalable architecture, and full regulatory compliance. Schedule a call with our DTx experts to discuss your product in-depth.
FAQ
Digital therapeutics products handle sensitive health data. That makes them subject to stricter privacy regulations (e.g., HIPAA in the United States). Securing regulatory approval typically requires ensuring privacy and data security, as well.
The DTx industry is far from mature. Stakeholders and customers alike lack awareness of the distinction between DTx products and wellness apps. Proving clinical value is also more difficult, albeit necessary for healthcare integration.
Common DTx startup obstacles during scaling include: Overcoming lack of awareness and resistance to new tech Differentiating from non-DTx healthtech products Balancing product updates with regulatory approval Meeting higher performance requirements
Real-world evidence is important because the effectiveness demonstrated during RCTs may not translate into real-world benefits. Working with real-world data is difficult because startups need to: Ensure data quality and accuracy Maintain patient privacy and data security Ensure the sample is representative and the data is complete
Connect with us
.webp)
We are a tech partner that delivers ingenious digital solutions, engineering and vertical services for industry leaders powered by vetted talents.





.webp)